A team of Scottish researchers has discovered that green LEDs could hold the key to extracting a valuable ingredient for natural health and wellbeing supplements from microalgae.
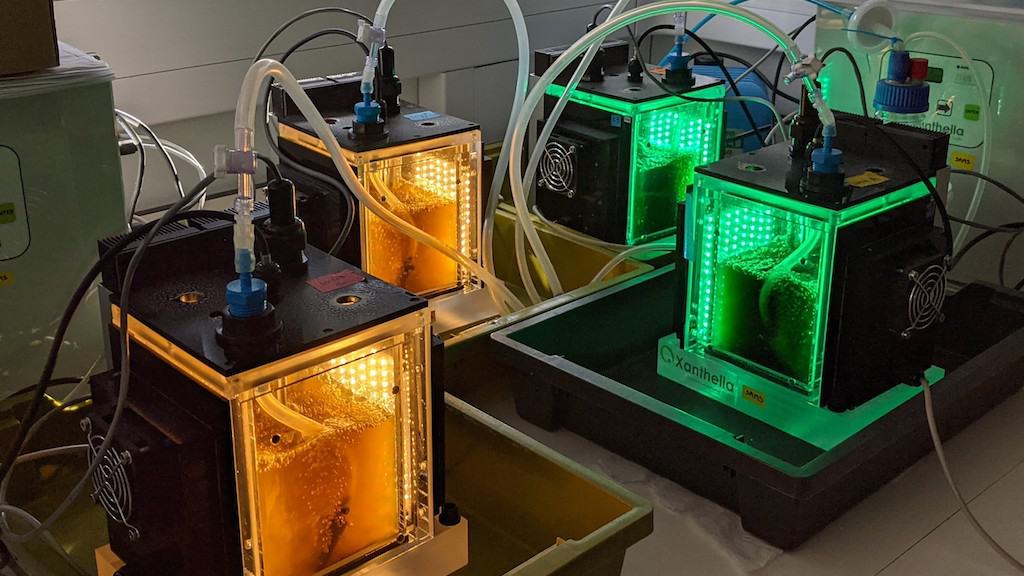
The feasibility study, funded by the Industrial Biotechnology Innovation Centre (IBioIC) with support from Algae-UK, was led by the Scottish Association for Marine Science (SAMS) and Xanthella, an Oban-based company specialising in photobioreactors – a type of technology that uses light to accelerate the growth of various microorganisms, including algae.
By exposing microalgae to a range of different colours of light, the project team found that green light, in particular, could enhance the production of fucoxanthin, a pigment commonly used for anti-inflammatory and antioxidant dietary supplements.
‘While we are still at a very early stage of what could be a new Scottish supply chain for fucoxanthin, the results of this study could have significant commercial benefits. Discoveries like this show the real value of applied research projects and we now have an opportunity to share what we have discovered with the wider algae and aquaculture sector. It has also led to further collaboration between SAMS and Xanthella and we are continuing to look at the impact of LED light treatment to influence and control the growth of microalgae for different purposes,’ said Professor Michele Stanley, associate director for innovation at SAMS.
The global market for fucoxanthin is still in its infancy, but is expected to grow to more than £245 million by 2027 and, while usually harvested from wild seaweed, the results of this project could lead to the development of an entirely new supply chain.
Researchers found that the highly targeted process, largely using green light, was much more efficient than using a standard photobioreactor settings and, therefore, required less energy to deliver significant growth. Manufacturing the pigment this way, instead of extracting it from seaweed, is also much more controlled, less impacted by seasonality and offers a more reliable level of output.
The study showed that around 20% of the treated microalgae could be extracted directly as fucoxanthin, with numerous opportunities to use the remaining biomass for other valuable purposes, such as biofuels, bioplastics or high-protein animal feeds.
‘It is widely understood that blue and red wavelengths of light are important for photosynthesis in plants, but it was surprising to see that green light could also boost growth in microalgae too. Using LED light, the aim of our photoreactors is to mimic other climates and recreate the conditions that cause algae to create different pigments, but with a much greater degree of control,’ commented Douglas McKenzie, chief executive of Xanthella.
‘In terms of next steps, we need to consider how the process can be scaled up effectively, determining the optimal conditions to create the largest proportions of high-value pigment from the least amount of biomass.’
As well as fucoxanthin, products derived from microalgal pigments are commonly used in a range of household and industrial products, such as spirulina, also used in diet supplements, and astaxanthin, a red-coloured pigment used in certain animal and fish feeds. Over the years, synthetic alternatives have been developed, but targeted microalgae production could open up a much more sustainable method of manufacturing them.
‘Innovation and collaboration can help us to improve Scotland’s sustainable manufacturing capabilities. The development of bio-based alternatives, such as those derived from microalgae, will be key to reducing our reliance on petrochemical-based products,’ said Liz Fletcher, director of business engagement at IBioIC.
‘More efficient production can also reduce our reliance on imports and we look forward to seeing what this study could lead to. Our ambition is to support the growth of the bioeconomy, and a key part of that is connecting research teams with industry partners, whom can work together to create a more sustainable Scottish economy.’









